Purina Recall 2024 Fda Guidance – Lifileucel is a one-time autologous adoptive cell transfer therapy that utilizes a tumor infiltrating lymphocyte manufacturing process. The BLA is supported by data from the C-144-01 study . The GCPG is the first of a series of compliance guidance anticipated to be issued by the OIG throughout 2024. The new guidance is intended to replace the existing fragmented (and generally out of .
Purina Recall 2024 Fda Guidance
Source : truthaboutpetfood.com
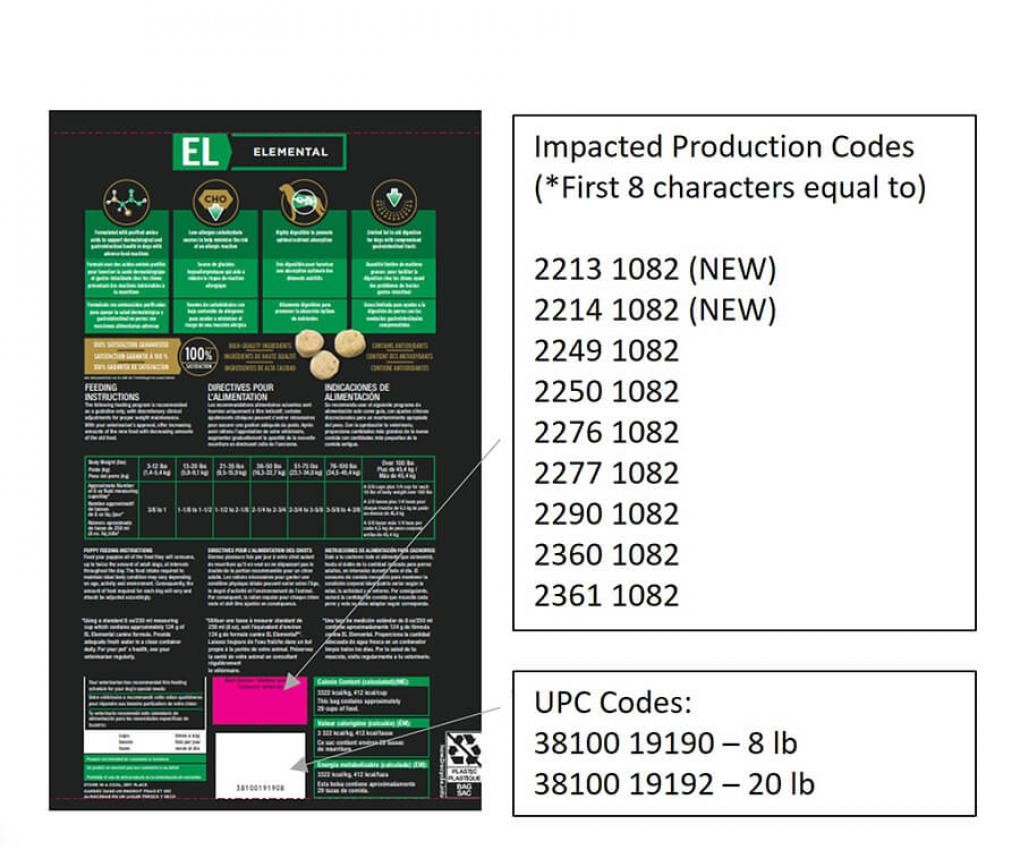
Labeling Error: Nestle Purina Voluntarily Recalls Limited Amount
Source : www.petage.com
Purina Recalls Pro Plan Vet Diet Product Due to Elevated Levels of
Source : www.dogfoodadvisor.com
FDA: Nestlé Purina Expands Voluntary Recall of Pro Plan Veterinary
Source : www.petage.com
Purina expands recall for dog food
Source : www.newsnationnow.com
Purina dog food recall over excessive vitamin D
Source : wgntv.com
FDA: Nestlé Purina Expands Voluntary Recall of Pro Plan Veterinary
Source : www.petage.com
Purina Pro Plan Veterinary Diets EL Elemental recalled over
Source : www.wxyz.com
Purina Recalls Pro Plan Vet Diet Due to Mislabeling
Source : www.dogfoodadvisor.com
Purina recalls some dog food due to possible elevated levels of
Source : www.wishtv.com
Purina Recall 2024 Fda Guidance Consumer Reports of Sick/Dying Pets Linked to Purina – Truth about : We are actively investigating adverse event reports in conjunction with local and state health departments,” the FDA said in a Facebook post on Wednesday. Neptune Resources, LLC has agreed to . ConsumerAffairs is not a government agency. Companies displayed may pay us to be Authorized or when you click a link, call a number or fill a form on our site. Our content is intended to be used .